
(for example, spread 2 x 104 cells per one well, then incubate in a CO 2 incubator for one night.) 2) Wash the slide 2 times with PBS. Briefly contrast and compare Western blotting versus immunostaining in tissue. SDS-PAGE & Western blotting.) Immunocytochemistry 1) Culture the cells in the appropriate condition on a glass slide. Describe the procedure for dot blot hybridization and compare this to western blotting. Detection is typically performed using chromogen or peroxide-linked secondary antibodies to catalyze a chromogenic or chemiluminescent reaction. The bases of western blot identification are two distinguishing properties: molecular weight and antibody binding specificity. What is the purpose of actin in western blotting Describe the major difference between Immunofluorescence and Western blotting techniques. This technique exploits the specificity inherent in antigen-antibody recognition.

This is followed by probing with antibodies specific to the protein being studied on the membrane, a method that is similar to immunohistochemistry, but without a need for fixation. The membrane can then be blocked with serum albumin or milk solution to prevent non-specific antibody binding. Membranes can be of the nitrocellulose, polyvinylidene difluoride (PVDF), or nylon variety. Protein binding to the membrane is an irreversible mechanism. As the proteins migrate out of the gel, they are captured on a membrane. for detection with chemoluminiscene using the ChemiDoc XRS+ System of Bio-rad laboratories. To achieve this, western blot implements three steps: (1) separation by size, (2) transfer to a solid support, and (3) visualizing target protein. Use the normal protocol for westen blot to detect your protein. In the electric field generated by a power supply, the proteins coated with negatively charged SDS migrate toward the positive electrode. Western blot allows us to determine the relative protein levels between samples and establish the molecular weight of the target, which can provide insight into its post-translational processing.
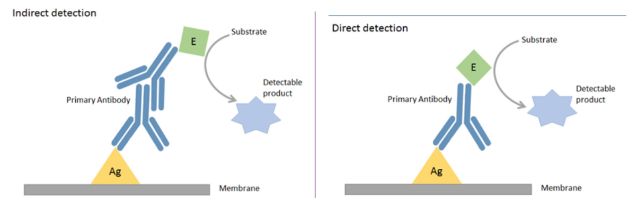
The Principle of Western Blot Western blot is performed by using polypropylene gel electrophoresis. After this, they are transferred to a synthetic membrane via dry, semi-dry, or wet blotting methods. For the accomplishment of the western blot, there are three elements, separation of proteins by size, transferring proteins to a solid support, and marking proteins by primary and secondary antibodies for visualization. Proteins are generally separated by size using sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis. This analytic technique proceeds in the following steps. Western blotting is a protein detection method using specific antibodies and involves two major processes: separation of soluble proteins into distinct bands. Immunoblotting Procedures Figure: Immunoblot: proteins separated by molecular weight and represented by a dark band on a blot.


 0 kommentar(er)
0 kommentar(er)
